Highlights of the Concept Paper on the revision of Annex 11
In December 2022, the Pharmaceutical Inspection Convention Pharmaceutical Inspection Co-Operation Scheme (PIC/S) and the European Medicines Agency (EMA) published a draft version of the Concept Paper on the revision of Annex 11, to cope with the progressive evolution of the life science organizations and their incremental shift to new technologies. Below, we will provide an overview of all the proposed new additions and changes and their impact on your business.
What is behind the update of Annex 11?
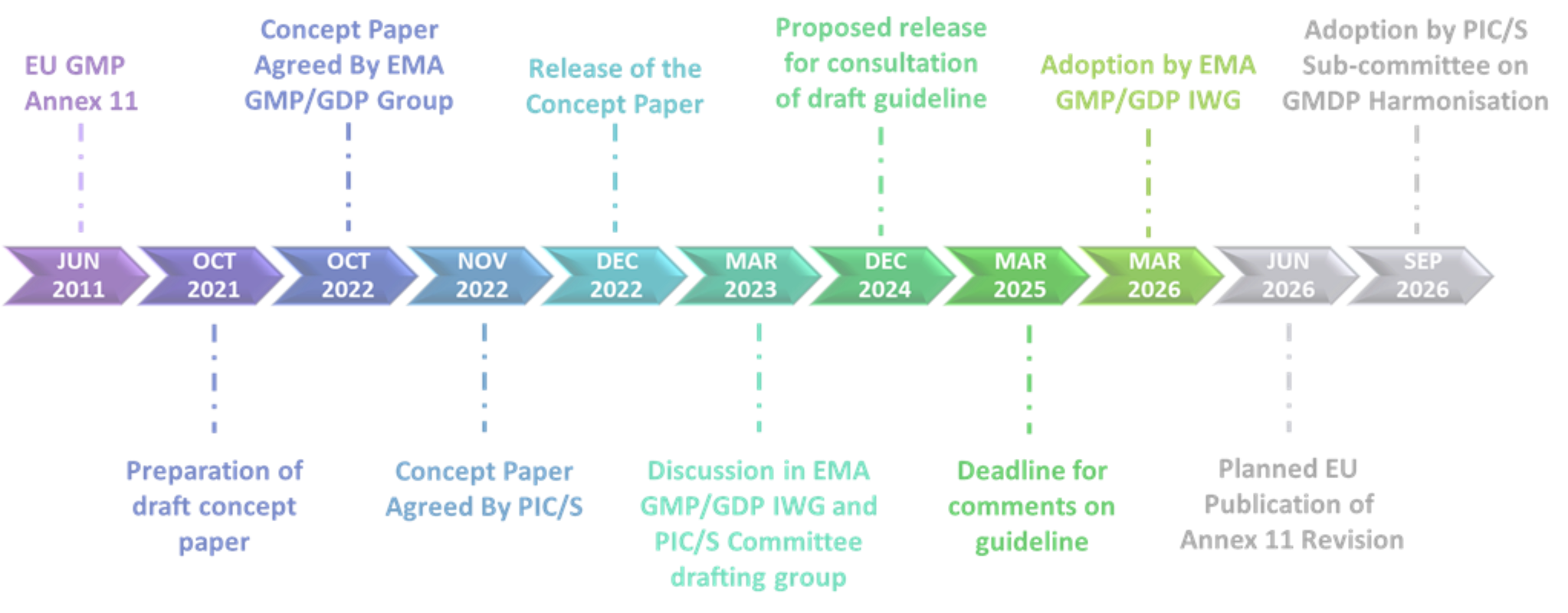
Annex 11 is part of Eudralex Volume 4 and refers to Computerized Systems. It offers guidance on the use of electronic systems involved in Good Manufacturing Practice (GMP) activities. Since the last release of Annex 11 in 2011, life science organizations have evolved, as has the use of agile methodologies, artificial intelligence, machine learning, service models such as cloud solutions, and the process of digital transformation. To address the progressive increase in the utilization of these new technologies and the new risks to which they expose, the concept paper has been released in December 2022, to provide appropriate regulatory guidance while ensuring compliance. The 33 points of the concept paper reflect the structure and chapters of the current version of Annex 11 (2011) and provide an overview of new points to be included and topics to be updated. Although changes are proposed in many chapters, other chapters remain virtually unchanged. The proposed timeline envisages the finalization and publication of the revised Annex 11 in June 2026 (Figure 1).
Figure 1 Proposed Timeline of Annex 11 Revision
Eudralex Volume 4: Annex 11 Computerised Systems and PE 009-15: Annex 11 – Computerised Systems will both be replaced by the updated guidance.
What is new?
The new additions promote the transition to digital transformation and the usage of integrated technical controls, which will be expected to play a more important role in ensuring reduced risk and improved compliance throughout the entire lifecycle of the computer system.
It also embraces the need to include regulatory guidance on the application of artificial intelligence and machine learning models in critical GMP applications, as well as for Agile development processes, also known as iterative approaches, which offer greater flexibility to change.
The increasing use of Cloud systems for GxP data storage and/or application deployment and the importance of choosing the right IT service provider such as Cloud Service Providers is another crucial point brought to attention. Indeed, a documented risk assessment must be conducted to consider the criticality of the application and its electronic records.
Interestingly, it also refers to the FDA´s draft guidance on Computer Software Assurance for Production and Quality System Software (CSA) published in September 2022, and the EMA GCP website, which provides guidance, through questions and answers, on good clinical practice (GCP).
Another hot topic is the Audit Trail, which covers no less than seven points in the concept paper. The proposed updates bring this section of Annex 11 closer to other existing regulations, proposing a more detailed and comprehensive guide, which reinforces the importance of a mandatory Audit Trail Functionality, which cannot be switched off, and an appropriate Audit Trail review frequency.
Last but not least is the Security chapter. The incremental use of technological solutions, such as cloud systems, can expose us to new risks, so it is necessary to guarantee the confidentiality, integrity, and availability of the data and the system, according to ISO 27001 (Information technology – Security techniques – Information security management systems – Requirements). Controls and authentication must only allow access to the system when the user is recognized with a high degree of certainty.
Conclusion
In conclusion, the new draft of the Concept Paper on the revision of Annex 11 aims to face and embrace the progressive use of new and more sophisticated technologies by updating and introducing new chapters.
If you are interested and want to find out more, please contact us.