ICH Q9 (R1) – What is new and how to navigate it
The ICH’s (International Council of Harmonisation) revised guideline ICH Q9 (R1) on Quality Risk Management (QRM) reached in the beginning of the year Step 4 of the ICH process. This means that the final revised document was adopted by the ICH Regulatory Members of the ICH Assembly (formal ICH procedure). While some of the Regulatory Members have already implemented the revised document (Step 5) and some others are still in the process of doing so, we decided to create a blog series focusing on the major changes that are brought by the revision. What do these changes mean in practice, which is their impact on the quality risk management process and what are effective ways to implement them?
ICH Q9 established a framework for QRM with the intention of introducing QRM approaches that could be leveraged by both the industry and regulators. However, it has been observed that the full potential of QRM has not been reached, which is the reason why the council decided on issuing a revision. The new themes serve as areas of improvement that can facilitate the implementation of QRM.
These include:
1. Formality in Quality Risk Management (5.1)
2. Risk-based decision making (5.2)
3. Managing and minimizing subjectivity (5.3)
4. The role of quality risk management in addressing product availability risks arising from quality/manufacturing issues (6.1)
Part 1: Formality in QRM
Understanding formality
The ICH clarifies within Q9 (R1) the term, as there is lack of understanding on what constitutes formality within this process. Lack of understanding leads to confusion and teams end up employing almost exclusively the most “formal” methods, such as FMEA, trying to remain compliant. This leads to decreased flexibility when it comes to QRM activities and might be at the expense of leveraging critical thinking throughout the process. Furthermore, going overly formal might lead to unnecessary reporting or resource utilization whereas in some cases smaller teams and documentation within existing tools might be sufficient.
Formality as a spectrum
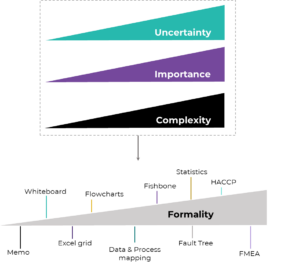
ICH points out that formality ‘is not a binary concept’, with either formal or informal methods but rather a gradient or ‘spectrum’. Within this gradient, methods have different degrees of formality which can be employed at different stages or activities of the QRM in a way that one can leverage their full potential based on the question to be answered in each case (Figure 1).
But how does this work in practice? How can one be certain on the level of formality needed for their specific QRM activity? ICH Q9 (R1) suggests considering 3 factors to decide on this matter:
✓ Uncertainty; defined here as lack of knowledge of the hazards and therefore associated risks of the area of the QRM activity. When the activity needs to be performed for example on a new system, never employed before in the company or department, this brings a high degree of uncertainty.
✓ Importance; what is the impact of the risk-based decision in question? Does it directly impact product quality or patient safety?
✓ Complexity; how complex is the system, area or process? Are there more systems or processes affected?
Figure 1: QRM methods with different degrees of formality should be chosen based on the assessment of uncertainty, importance and complexity of the activity in question. Figure inspired and modified from Peter Baker
Formality decisions and how to document them
After considering these factors, one needs to determine how this can be ‘translated’ into the choice of a method with the appropriate degree of formality (see Figure 1). The general recommendation by the ICH is to rely on more formal methods when the degree of the 3 factors described above is high and go with lower formality for activities where greater certainty, lower impact and lower complexity exist. Of course, a combination of methods for the different stages of the activity is also a possibility, as suggested by Peter Baker within his blog post on this topic.
There is no doubt that the process for reaching a decision regarding formality shall be documented and integrated within the QMS (Quality Management System) and so is the case for the outcome of this assessment.
An official quality document such as a QRM SOP, can serve as a good guide to help teams reach appropriate conclusions. Visualization tools such as process and/or data mapping can also be applied to facilitate the identification of inputs, outputs and existing control measures. They are recommended by ICH Q9 (R1) (Annex I) as simple techniques to structure risk management and companies can benefit in multiple ways from their application as they enable the definitions and understanding of the business process; the starting point for all activities of a regulated company.
At last but not least, regulated companies should be ready to demonstrate the existence of a systematic approach for the integration of QRM principles within their operations, including decisions regarding formality, as reflected within the PIC/S Aide-Memoire on Assessment of Quality Risk Management Implementation.
Don’t forget, what is not documented, never existed in the first place!
Stay tuned for the next articles within this ICH Q9 blog series series where we will discuss the new additions to the revised document.
Also do not hesitate to contact us if you have questions about enhancing your QRM process or about deciding on QRM activities.