Navigating the computerized system landscape on the path to laboratory digitalization
Laboratories in the life science industry face challenges in the implementation, setup, and inter-connection of suitable laboratory computerized systems. The increasing number of vendors and systems, as well as complex individual demands complicate or slow down efficient digitalization in the laboratory.
How can you know which options will serve your business best?
It is crucial for life science companies to define and map their processes and areas for which a computer system shall be implemented or exchanged to benefit optimally from these systems’ potential in standardization and simplified management of workflows, laboratory processes, documentation, and quality procedures. By understanding internal demands, identifying suitable systems, and structuring interface implementation accordingly, life science companies can reduce cost and effort during the digitalization process with laboratory computer systems.
Assuming you have already investigated the demands the system should fulfill, understanding the landscape of systems available is your next step in a structured approach for system implementation.
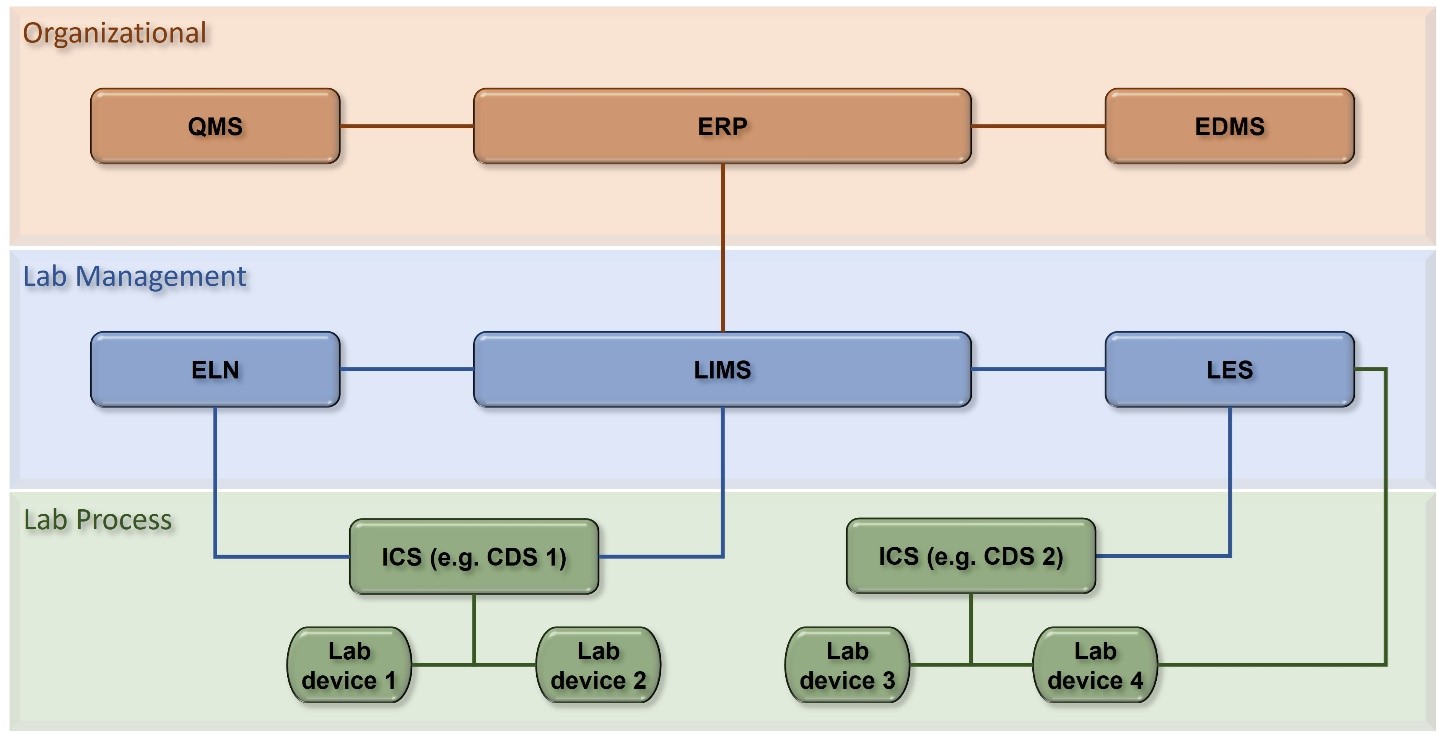
Here, we categorize core laboratory computer systems into three levels, “organizational”, “laboratory management”, and “laboratory process”. An overview of key systems at each level and visualization of an exemplary system interface map (Figure 1) can assist the identification and selection of suitable options according to individual requirements.
Figure 1: Example of computer system setup and map of connections. Computer systems in the laboratory are laid out in three categories according to their use: Overall organization, laboratory management and laboratory process. At the heart of organizational management is the ERP. It enables most cross-company processes feeding data to quality systems like QMS and EDMS but also to the systems of the category laboratory management. As the central system, ERP also receives data and information from the other systems. On the laboratory management level, LIMS is the central system to organize and manage laboratory processes, workflows, and samples. While LES and ELN are involved more closely with laboratory data and equipment, they can also be implemented features of a LIMS. On the laboratory process level, ICS’ such as a CDS are used to control laboratory devices and analyze the recorded data. While ERP, LIMS, and ICS take key positions, implementation of additional systems and their inter-connection is unique to the individual demands of a life science company, unit, or laboratory.
Let’s take a closer look at the systems that make up these three levels.
Organizational computer systems are non-laboratory specific and are used for diverse functions across different company units.
- An Enterprise Resource Planning (ERP) (e.g. SAP S/4HANA) software program is the heart of most companies. It is designed to manage and integrate all aspects of operations, including inventory management, production planning, financial management, and supply chain management from raw materials to finished products. An ERP can function as a centralized database for all laboratory data and can provide a structured approach to laboratory management, ensuring consistent processes and quality control standards.
- The Quality Management System (QMS) (e.g. Sparta Systems TrackWise) is used to manage and monitor all quality control processes, including change control, deviation management, and document control. It ensures that laboratory processes are consistent and that quality control objectives are met. The QMS can be set up independently or can be a sub-functionality of an ERP.
- An Electronic Document Management System (EDMS) (e.g. Veeva Vault) functions as a central database for all laboratory documents and records. It includes for example standard operating procedures (SOPs), batch records, and product specifications. An EDMS allows for easier management of laboratory documentation and information retrieval, as well as ensuring compliance by its secure and auditable approach to document management. The EDMS can be an integrated part of a QMS, an ERP, or be set up as a standalone system.
Laboratory management computer systems facilitate laboratory workflows, efficient documentation, and supplying standardized procedures.
- A Laboratory Information Management System (LIMS) (e.g. LabWare LIMS) is the lab-specific counterpart to the general organizational ERP. A LIMS is the central laboratory software to manage workflows, track samples, generate reports, and analyze data. It allows for efficient and organized control of all laboratory processes thereby enabling to optimize resources and streamline laboratory operations. The built-in quality control features such as audit trail, role-based user access management, and e-signatures help to reduce errors and comply with regulatory requirements.
- A Laboratory Execution System (LES) (e.g. iVention iLES) is designed to control and monitor laboratory processes in real-time. By directly interfacing with laboratory devices, the LES can automatically document parameters and data and follow the SOP as the equipment is running. LES enables labs to improve efficiency, reduce errors, and automate and streamline processes.
- An Electronic Laboratory Notebook (ELN) (e.g. SciNote ELN) is a software program designed to capture, manage and organize laboratory data, document experiments, and record results. In contrast to a paper-based laboratory notebook, an ELN provides a secure and centralized database for the documented data, making it easier to manage and retrieve information, share data, and allow collaboration between staff. Thereby, an ELN has the potential to increase the efficiency of all laboratory procedures. With built-in quality features, ELNs help to improve data integrity and ensure quality compliance.
Laboratory process computer systems directly control laboratory devices or provide specialized analysis tools for specific devices and methods.
- Instrument control software (ICS) controls laboratory instruments such as spectrometers, chromatographs, and balances, ensuring that they are calibrated correctly and producing accurate and reliable results. ICS helps to reduce human error and ensures that instruments are performing optimally. Via real-time monitoring of instrument performance, ICS allows laboratory staff to identify issues and take corrective action.
- Chromatography Data System (CDS) (e.g. Thermo Scientific Chromeleon CDS) is an example of an ICS that additionally includes features of a data analysis tool. CDS controls chromatography instruments such as high-performance liquid chromatography (HPLC) and gas chromatography (GC). It is designed for acquisition, processing, and reporting of chromatographic data and results. Automated data analysis with a CDS increases method efficiency, reduces human errors, and improves data quality.
In summary, each type of laboratory computer system suits specific needs and interfaces with other systems on different levels. “Laboratory process” systems, such as CDS, control laboratory devices, record data, and help analyze results. They are interconnected with “laboratory management” systems, which document results and data (ELN), and manage workflows (LES), processes, and samples (LIMS). In turn, they are connected to “organizational” computer systems that manage resources (ERP) and quality documentation (QMS). While these digital systems function on different levels of laboratory processes, they are all intertwined in enabling standardized quality procedures and compliance with regulatory requirements to ultimately maintain the safety, efficacy, and quality of products.
Ultimately, each laboratory computer system has the potential to improve work processes in the laboratory. However, why should life science companies take on the high effort required for the correct implementation of so many systems?
In our opinion, and according to the guidance of many regulatory agencies, the benefits far outweigh the burdens. An efficient setup of laboratory computer systems and interfaces allows to streamline operations and improve data integrity. By providing a structured and organized approach to laboratory management, these systems ensure that laboratory processes are consistent, while reducing manual work effort. Implementing automated processes controlled by laboratory computer systems has the potential to reduce costs and human errors, as well as increase overall efficiency.
Due to this high pay-off, it is well worth it for life science companies to overcome the complex, and time-intensive challenges in setup and implementation of suitable laboratory computer systems and interfaces. The continuous benefits far outweigh and outlast the initial effort.
If you have questions or challenges regarding laboratory computer systems, from general information via implementation to process validation, reach out to us!