Think Beyond the Device – The New Age of Analytical Qualification
Why USP <1058> is so important in the world of GMP
Modern laboratory equipment is becoming increasingly complex. This poses new challenges for companies in GxP-regulated environments: How can you ensure that equipment measures reliably, data is accurate, and regulatory requirements are met? This is exactly where USP <1058> comes into play.The current revision of the directive places greater emphasis on risk-based qualification, the integration of software-based systems, and a comprehensive life cycle approach. This not only ensures technological safety but also strengthens data integrity, product quality, and patient safety.With USP <1058>, companies can ensure that their devices are GMP-compliant, accurate, and officially recognized – a key part of quality assurance in the laboratory.
What is changing in USP <1058>?
With the end of the public comment period on May 31, 2025, the draft of the revised USP <1058> Draft enters the next phase. The revision of this guideline for the qualification of analytical systems affects a wide range of professionals, from laboratory managers to equipment manufacturers. But what exactly is changing?
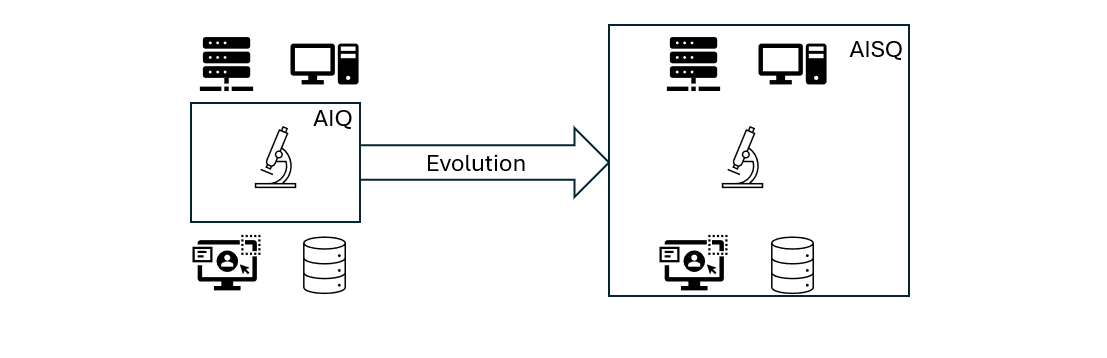
1. From AIQ to AISQ
In the past, qualification was only about devices (AIQ – Analytical Instrument Qualification). Today, qualifications also include software, networks, and entire systems. Modern devices are often software-controlled – firmware, control programs, and data management solutions are now central to functionality.
2. Life cycle approach
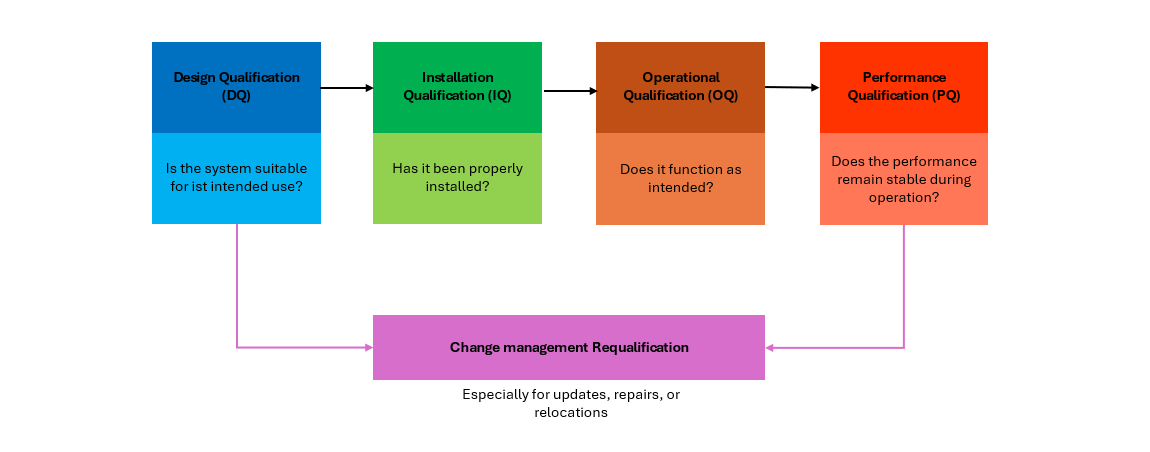
Qualification is now a continuous process, divided into three phases:
- Specification & selection
- Installation & performance qualification
- Continuous monitoring
The classic 4Qs are not being abolished but integrated into this new model.
3. Risk-based approach
Not every measure is equally important. The intensity of qualification depends on the risk to data integrity and product quality.
4. Measurement uncertainty
New: Measurement uncertainty is now also considered when evaluating device performance.
5. Extended software classification
Software is classified in greater detail, including new subgroups for complex systems (Group C).
6. Traceability and change control
- Traceability matrix for necessary software
- Stricter change control: Updates, relocations, or repairs must be systematically evaluated and documented.
7. Clear roles and responsibilities
Tasks between users, IT, QA, and suppliers are clearly separated and documented.
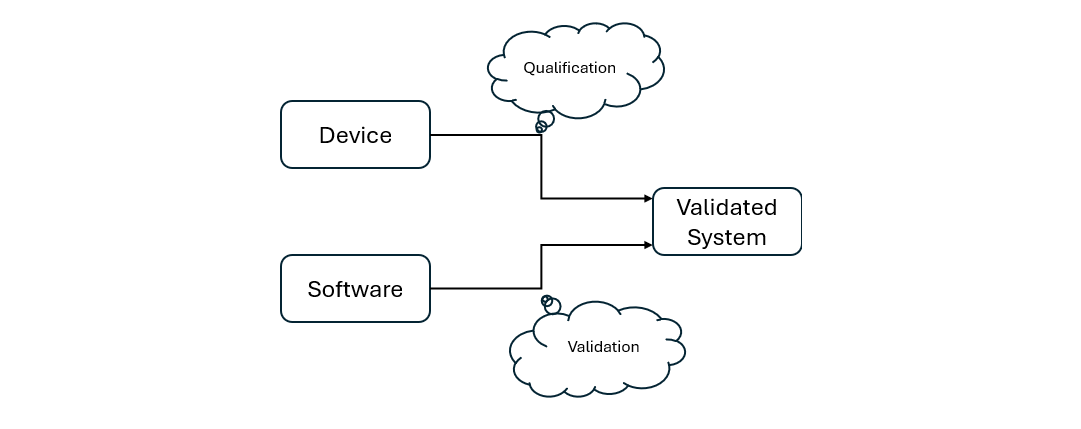
AISQ: Devices and software working hand in hand
The new approach requires hardware qualification and software validation (computerized system validation, CSV) to work together. Issues such as user management, audit trails, and data security are becoming increasingly important – a step toward a holistic system approach.
Example from the laboratory: A device suddenly reports unusual values. Thanks to AISQ, both the hardware and software are checked – errors are detected and documented at an early stage.
If you would like to find out more about CSV, take a look at our website.
Data integrity – the basis for reliable decisions
Data quality is crucial. Data integrity (DI) ensures that all data is complete, traceable, and tamper-proof – from recording to archiving. This is the only way laboratories can make valid decisions and meet regulatory requirements.
If you would like to find out more about Data integrity, take a look at our website.
Conclusion: AISQ – The future of laboratory qualification
The new version of USP <1058> goes beyond a mere update. With AISQ, modern laboratories can meet growing requirements. Those who implement the guideline not only ensure compliance but also lay the foundation for stable, valid analysis results.
How does GxP-CC support you?
We help you qualify your laboratory equipment following USP <1058> – in a practical, compliant, and documented manner. Whether risk-based classification, qualification plans, or GMP-compliant test protocols – we go with you every step of the way. Efficient, audit-ready, and individually tailored to your requirements.
If you would like to learn more or discuss your specific needs, feel free to contact us via our website.