GCP-IWG 2021 Annual Report Reveals Critical Findings in Clinical Trials Quality and Integrity
The Good Clinical Practice Inspectors Working Group (GCP-IWG) ensures clinical trials follow EU standards. The group recently published their Fourteenth Annual Report for 2021 on November 21, 2022 – just six months after their 2020 report which was delayed because of COVID-19.
Although this report includes fewer activities than previous years due to the impact of the COVID-19 pandemic, the group remained committed to prioritizing the most critical GCP-related activities during this challenging time.
This article discusses the major areas of concern and findings outlined in the report.
Weaknesses and Critical Findings
In the period of 2021, the inspectorates carried out a total of 27 triggered GCP inspections. One inspection from 2019 and few more from 2020 were not performed due to Covid-19 restrictions and were postponed to 2021, therefore were included in this annual report.
From the total amount of inspections, 33,3% were conducted in the EU/European Economic Area, followed by 25,9% in Asia and 18,5% in Eastern Europe-Non EU.
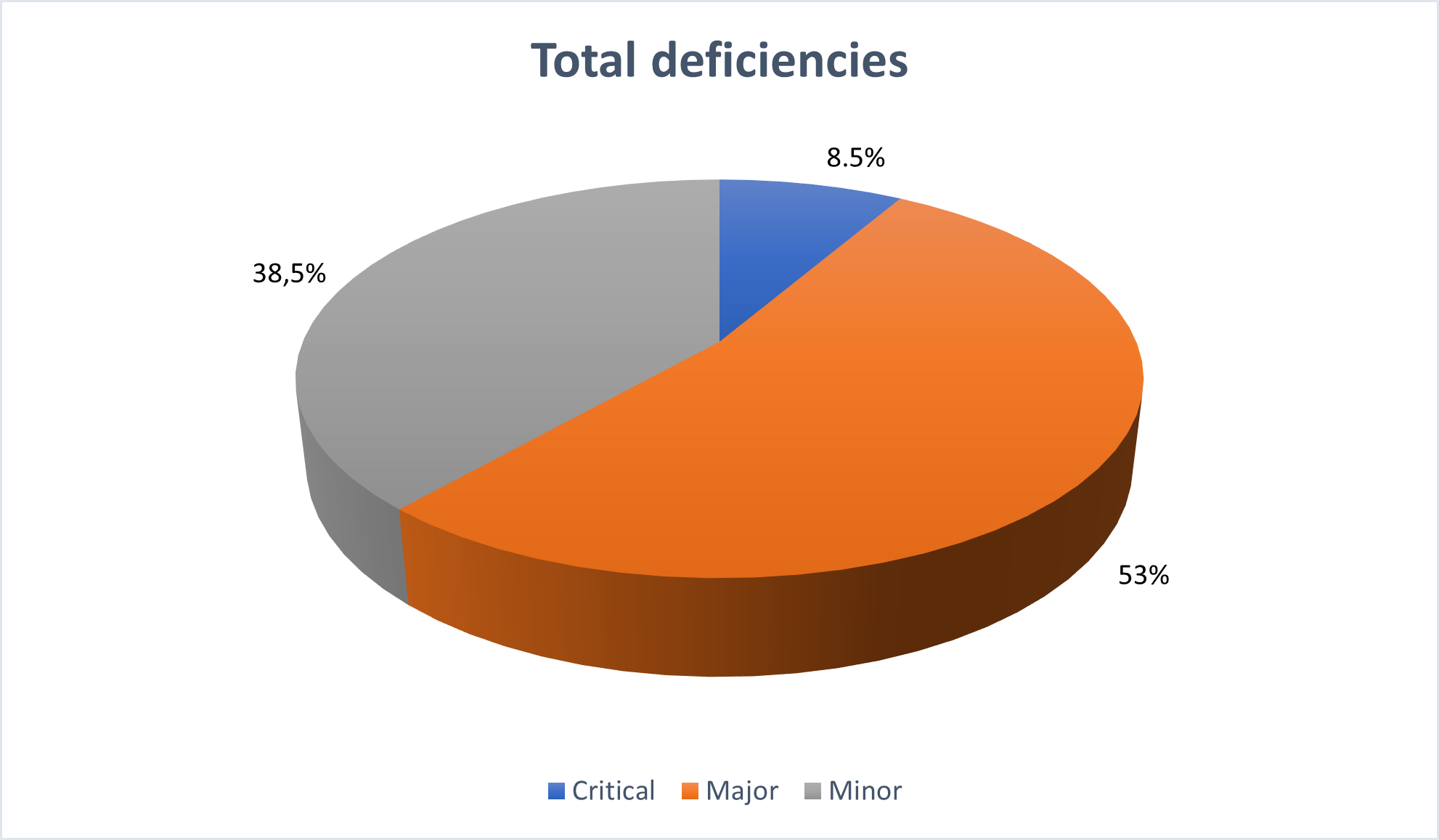
A total of 286 deficiencies, comprising 24 critical (8,4%), 152 major (53,1%) and 110 minor (38,5%) findings were recorded for the 27 inspections conducted in 2021, requested by CHMP (Committee for Medicinal Products for Human Use. On average, each site inspected had 10-11 findings.
Top three categories of deficiencies
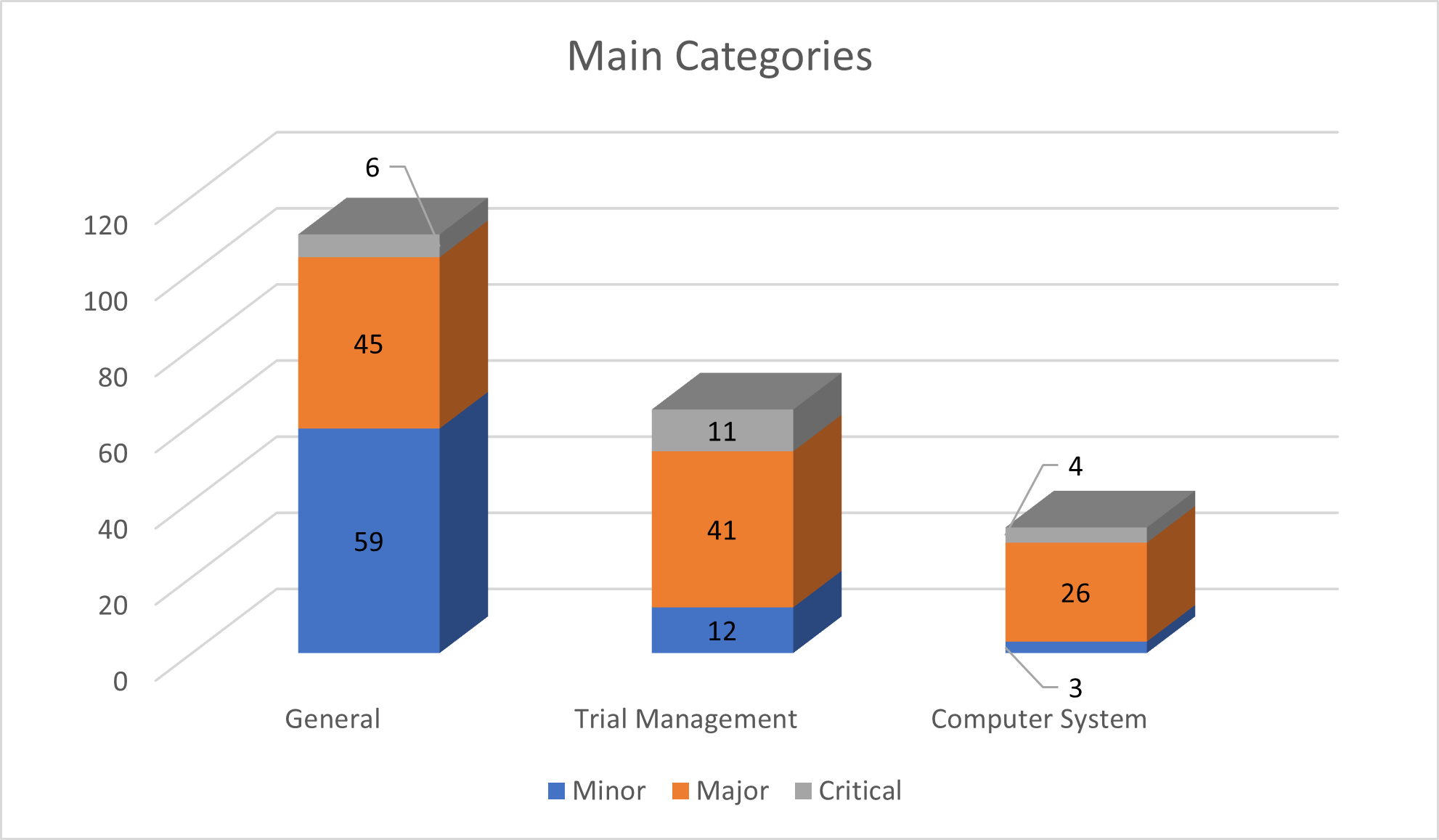
During the inspections, many categories of findings were identified. Among these categories, the three most significant ones with findings were:
- General
- Trial Management
- Computer System
The GCP inspectors found six critical issues in the “General” category.
Three issues pertained to delays and missing documents related to essential documents. Two issues were related to organization and personnel, such as unclear arrangements and delays in changing the principal investigator. Finally, one issue was related to the lack of SOPs for critical processes.
The Trial Management category had eleven critical findings, with six related to Data Management, including PI not reviewing and signing off on eCRF data, and backlog in Source Data Verification. Three findings were related to Protocol / Case Report Form / Diary / Questionnaire design, such as protocol deviations due to lack of clarity. Another two findings were related with Clinical Study Report (CSR) and Statistical Analysis.
Lastly, let us focus on the third major category, Computer System, which had four significant findings. Two critical findings were related to Audit Trail and Authorized Access, highlighting the importance of having proper procedures in place for user access and audit trail to reconstruct events.
If you’re interested in learning more about this, check out the Data Integrity in GCP Part 2 – Case Studies – GxP-CC, where we provide valuable insights on the importance of audit trails and access control in ensuring data integrity, which is crucial for regulatory compliance and product quality.
The other two major findings related to Physical Security System and Backup, including issues such as lack of encrypted channels for remote access and inadequate periodic firewall reviews. For more on cybersecurity and regulatory compliance, see Quick Starter Controls to Reduce Risks on Industry 4.0 Implementations – GxP-CC.
Rising Deficiencies in Computer Systems
Interesting to observe is that there has been an increase in the number of issues found in computer systems category compared to the previous reports. This highlights the growing importance of proper procedures in place, conducting risk assessments and validation of computerized systems. Regular reviews and assessments of computer systems are necessary to identify and address potential vulnerabilities and risks.
Conclusion: The Need for Adherence to Guidelines and Regulations for High-Quality Clinical Trial Data
In conclusion, the report’s findings emphasize the crucial role of maintaining the integrity and reliability of data generated during clinical trials, as any compromise can potentially impact the safety and effectiveness of investigational products.
Alongside this, ensuring patient safety is a top priority in clinical trials, and the reliability of the data generated plays a critical role in achieving this goal. To maintain the quality, integrity, and security of clinical trial data, it’s imperative for sponsors and investigators to adhere to the latest guidelines and regulations. By doing so, we can be confident that the information gathered from clinical trials is trustworthy and can be used to enhance patient health.
Don’t hesitate to contact GxP-CC for assistance in navigating the complex landscape of clinical trials and ensuring that your data meets the highest standards of accuracy, reliability, and confidentiality. Together, we can work towards improving product quality and patient safety.