Part 4: Addressing Product Availability Risks
ICH Q9 (R1) – What is new and how to navigate it
Part 4: Addressing Product Availability Risks
Medicinal product shortages and limited drug availability is a global problem with multifactorial causes that brings significant challenges to the healthcare systems and impacts patient health. Many regulatory agencies have initiated strategic initiatives as well as collaborations to prevent and tackle drug shortages which reflects the gravity of this issue. The EMA has established the Medicine Shortages Single Point of Contact (SPOC) working party but also a task force on availability of authorized medicines in collaboration with the Heads of Medicines Agencies (HMA). The FDA has implemented a respective taskforce as part of the Food and Drug Administration Safety Act (FDASIA) with the goal, among others, to enhance the safety of the drug supply chain. Within the scope of FDASIA, the Center for Drug Evaluation and Research (CDER), uses a variety of tools to monitor the supply chain with the goal of preventing and mitigating drug shortages.
Within the scope of the ICH Q9 revision (R1), the council addresses this problem from the perspective of drug quality or manufacturing issues. By introducing a new section within the updated document, dedicated on availability risks, the goal is to enhance the understanding of how proactive risk-based strategies can prevent or manage drug shortages by safeguarding product quality.
Patient protection
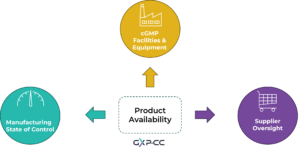
Protecting patients is the primary principle of QRM and this is reinforced by evaluating risk to quality with refence to patient safety. Patient safety is not only at risk when a drug of poor quality reaches the market but also when a drug is not available in the market because of quality issues during manufacturing or distribution. We are going to approach this topic by focusing on the following key areas, as described by the revised ICH Q9:
1. Process variability and state of control
2. Facilities and equipment
3. Oversight of outsourced activities of suppliers
Figure 1: Product quality and therefore quality-related drug availability is maintained by ensuring manufacturing operations, facilities and suppliers are managed based on QRM principles.
Manufacturing process variability and state of control
Robust processes within (c)GMP environments safeguard result reliability and product quality. In the absence of appropriate controls during the manufacturing process, errors can occur which can result in variable results or even to compromised product quality. Process variability leads to variable or out of specification test results requiring further testing which at best can delay product release or even result in poor quality products that can not be released to the market. In either case, the outcome is the same; a drug not available to the patient.
By designing manufacturing processes based on appropriate Quality Risk Management (QRM) approaches, one can identify hazards and associated risks and come up with controls to mitigate them and therefore ensure robustness. As discussed within the first article in our blog series on the topic of formality the choice of the appropriate QRM method should be based on the level of complexity, uncertainty and importance of the respective process. Complex manufacturing processes that entail a high risk to product quality and therefore availability, require QRM process approaches at the formal side of the formality spectrum. The “closer” the process is to the product, the more direct the impact to the quality and the higher the risk. However, even if a process is indirectly linked to product quality, when gone wrong, it might lead to manufacturing delays, hence product availability remains at risk. Therefore, such processes should be approached with higher formality (e.g., FMEA) where individual risks can be assessed and mitigated by acknowledging all the respective parameters.
Manufacturing Facilities and Equipment
Although a robust process is of great importance for ensuring that a pharmaceutical product is manufactured in a reproducible manner with high quality, so are the facilities and equipment or systems which are employed for implementing the manufacturing process. Designing and using facilities but also purchasing equipment according to cGMP US FDA (current Good Manufacturing Practices) and GMP EMA, based on QRM principles, can prevent production problems leading to supply issues.
Designing a new facility requires highly formal QRM processes to assess possible designs and evaluate if they comply with regulatory standards. Using tools such as FMEA and Ishikawa Diagrams while including experts of different fields that are knowledgeable about QRM processes, can ensure that risks will be assessed based on current knowledge. Such approaches facilitate the implementation of quality by design based on QRM principles. Likewise, new equipment should be purchased based on its intended use, while acknowledging the process. Such activities shall begin by defining requirements following a process risk assessment by a team of users and SMEs. To the extent possible, stakeholders shall aim at embracing digitalization and automation when designing facilities and purchasing new equipment as technology can reduce variability and the possibility of human error while ensuring data integrity.
Furthermore, QRM principles shall be applied throughout the lifecycle of the facilities and equipment to evaluate and mitigate risks on an ongoing basis but also assess the effectiveness of already implemented controls.
Supplier Oversight
With increasing supply chain complexity, it is more important than ever to maintain risk-based control over suppliers. Greater effort should be dedicated to suppliers whose products or services impact the quality of the drug products. Effective supplier oversight begins by applying QRM during qualification and approval, continues with ongoing monitoring and review and concludes by documenting the lessons learnt and overall supplier experience. Establishing and maintaining supplier lists in which suppliers are categorized based on their respective risk can be a useful tool to have in place.
When assessing suppliers, companies should employ Knowledge Management to make appropriate risk-based decisions (Blog post #2 on Risk-Based Decision Making) and establish adequate controls which need to be documented within the respective service contracts/agreements. Furthermore, the QRM approach of the supplier needs to be evaluated- as part of the overall QMS (Quality Management System) evaluation- to assess its effectiveness and potential impact to the provided product or service. Establishing risk-based communication and collaboration pathways with the suppliers and service providers is also important for effective oversight and risk control.
We hope that this article series (Part 1, Part 2, Part 3) with focus on the new topics of the ICH Q9 (R1) was interesting and made you think of ways to make your QRM process more efficient. In case you would like some help with that, do not hesitate to contact us.